ECONOMIC IMPACT.
The business opportunity for Prisma is huge. The system will compete in the wearable medical devices market, which was valued USD 13 billion in 2019 and is expected to exceed USD 90 billion by 2027, growing at a CAGR of 27.9% (Grand View Research).
It represents one of the fastest growing markets, propelled by people’s changing lifestyle, increasing prevalence of pathologies like obesity and diabetes, and rising availability of new technologies to treat chronic diseases.
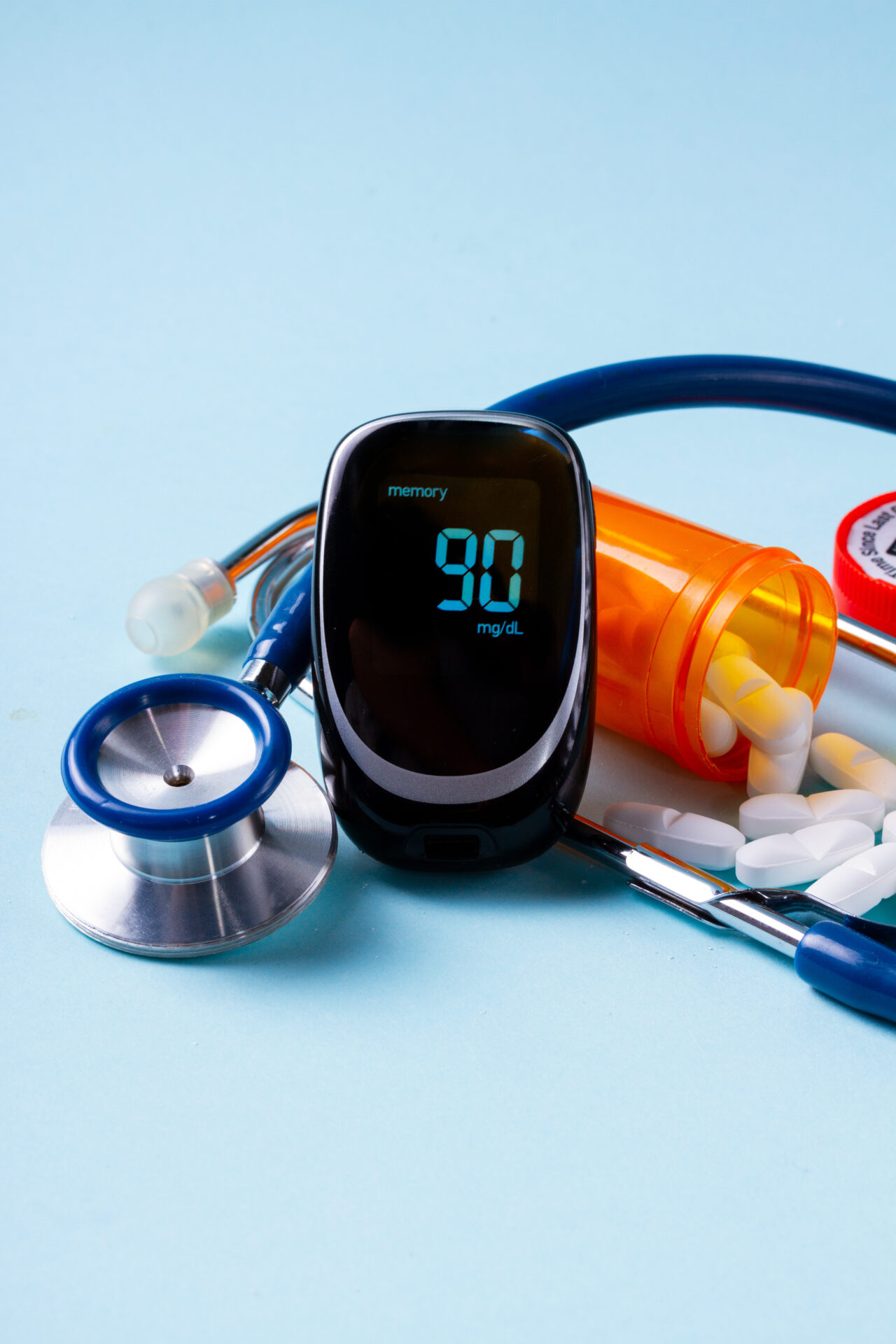
Diabetes is a major global problem, directly responsible for more than 1.6 million deaths, and other 2.2 million due to high blood glucose levels. It is estimated that around 420 million people are affected by this disease globally, with an incidence of about 8.5% of the adult population. The introduction of a the new PRISMA device could have a huge impact both on life expectancy and European health systems on diabetic patients (in countries where healthcare service is private), as it is poised to minimize the economic costs associated with diabetes complications, such as heart, kidney and eye diseases.
SOCIETAL BENEFITS.
The societal benefits deriving from the successful introduction of the Prisma innovation are relevant, as more than 420 million people affected by diabetes could improve their quality of life and efficacy of the therapy, which huge impact on productivity, life expectancy and reduction of complications and side effects, as well as increasing the compliance to the therapeutic regime.
In this context, it is important to notice that Type 1 diabetes is becoming increasing prevalent in children, who are also the segment of the population which shows the least compliance with the standard therapeutic approach2, and who will benefit from the adoption of a closed-loop system as Prisma.